Revealing Aspects of Cardiac Function from Fluorescence and Electrophysiological Recordings
ABSTRACT
This dissertation deals with three topics in the field of cardiovascular research. First, the dissertation deals with confocal microscope image enhancement. Second, diffusion restrictions in a single cardiomyocyte is studied. And third, different calcium fluxes in a single cardiomyocyte are determined with electrophysiological techniques. All three topics include experimental measurements, mathematical analysis and modeling. Fluorescence imaging has a central role in the dissertation.
With the help of image enhancement one can gain more information than is visible from raw data at first sight. One of the aims of this study was to improve the Richardson-Lucy deconvolution algorithm with total variation regularization for image enhancement. There are two major issues with the original algorithm: 1) it depends on a certain free parameter that determines the quality of enhancement but the choice of its value lacks definite criterion; 2) it lacks a reliable stopping criterion that has a great importance in balancing between noise reduction and detail enhancement. In this study a solution to these issues are provided. For that, a formula was derived to compute the parameter value during the iteration process. As demonstrated on the analysis of synthetic images, the algorithm yields equally good results as with optimal parameter value. Moreover, the analysis showed that the evolution of the parameter value can be effectively used as stopping criterion.
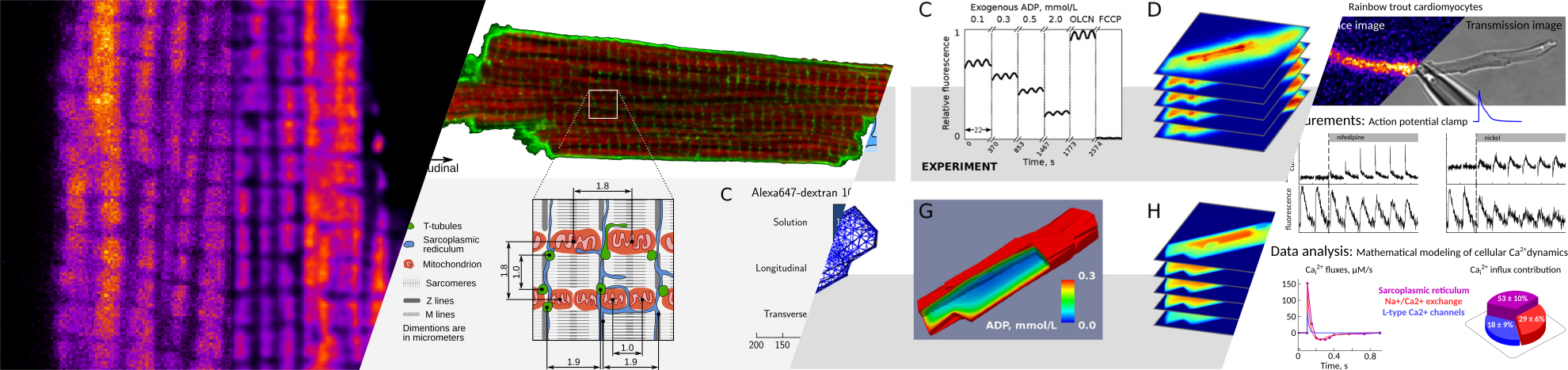
Deconvolution of confocal images is used to visualize the intracellular structures that may form diffusion barriers. These barriers govern intracellular energy transfer, which is crucial for proper cardiac function. There are two types of diffusion obstacles that interfere with energy transfer from mitochondria to ATPases: cytoplasmic diffusion barriers grouping ATP-producers and -consumers, and MOM with VDAC permeable to small hydrophilic molecules. Such profound diffusion restrictions are expected to play a major role in energy transfer, signaling, distribution of apoptotic factors and reactive oxygen species. Another aim of this work was to establish the partitioning of intracellular diffusion obstacles in cardiomyocytes. To determine the diffusion coefficient, partitioning of intracellular diffusion obstacles, and \mom permeability, two supplemental approaches based on fluorescence imaging and mathematical modeling were used. In the first method, raster image correlation spectroscopy was applied to determine diffusion coefficients of two fluorescently labeled molecules that differ in size. The analysis of the obtained experimental data revealed that in rat cardiomyocytes diffusion of smaller molecules is relatively more restricted than that of larger molecules compared with the surrounding solution. This counterintuitive result can be explained with diffusion restrictions in the cell consisting of semi-permeable barriers that form a regular lattice-like structure. The second method is based on the analysis of permeabilized cardiomyocyte mitochondrial response to different concentrations of extracellular ADP through the measurement of NADH autofluorescence. The results suggest that in permeabilized rat cardiomyocytes a small number of opened VDACs contribute only to half of the observed diffusion restriction between mitochondrial inner membrane and the surrounding solution. The other half of the overall diffusion restriction is clearly positioned in the cytosol on the way between the solution and the MOM.
The diffusion barriers near MOM and in the cytosol are essential for an adequate regulation of mitochondrial ATP-production. This, as well as mitochondrial metabolic capacity, seems in turn to be connected to ECC. In cardiac function, Ca has a central role in ECC, where changes in intracellular Ca concentration regulate contraction. The changes in intracellular Ca concentration are caused by a precise balance between different cellular Ca fluxes. In case of heart failure, this balance is altered, which results, for example, in a diminished cardiac output. The aim of the last topic of the thesis was to develop a robust method to quantify Ca fluxes in cardiac ECC. To determine the kinetics and contribution of different Ca fluxes a mathematical model of Ca-dynamics that combines the electrophysiological measurements of transsarcolemmal Ca currents during action potential clamp and their impact on the Ca mediated fluorescence transients was applied. With the aid of the novel method the contributions of different Ca fluxes in trout ECC were determined. Also, a significant role of sarcoplasmic reticulum in trout ECC was discovered, which is in sharp contrast with previous estimates. Moreover, the method is not trout specific and is applicable to other species as well.
In summary, the results of the dissertation represent broad range of research on different aspects of cardiac functioning, wherein substantial contributions have been made in the advancement of existing methods as well as in the development of novel methods.
SUPERVISORS: Marko Vendelin, Pearu Peterson, and Rikke Birkedal
OPPONENTS
- Prof. William Edward Louch, PhD; Institute for Experimental Medical Research, Oslo University Hospital and University of Oslo, Oslo, Norway
- Prof. Pasi Tavi, PhD; Department of Biotechnology and Molecular Medicine, A.I. Virtanen Institute, University of Eastern Finland, Kuopio, Finland
TIME OF DEFENSE
14 December 2016 at 10:00 in the Institute of Cybernetics, room B101
THESIS
You can download PDF of the thesis here