Jepihhina N, Beraud N, Sepp M, Birkedal R, Vendelin M
Biophys. J. 2011 Nov;101(9):2112-21
PMID: 22067148
Abstract
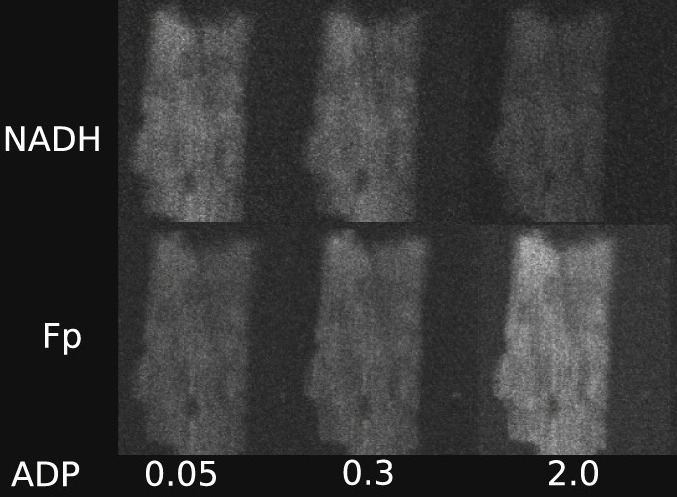
Intracellular diffusion restrictions for ADP and other molecules have been predicted earlier based on experiments on permeabilized fibers or cardiomyocytes. However, it is possible that the effective diffusion distance is larger than the cell dimensions due to clumping of cells and incomplete separation of cells in fiber preparations. The aim of this work was to check whether diffusion restrictions exist inside rat cardiomyocytes or are caused by large effective diffusion distance. For that, we determined the response of oxidative phosphorylation (OxPhos) to exogenous ADP and ATP stimulation in permeabilized rat cardiomyocytes using fluorescence microscopy. The state of OxPhos was monitored via NADH and flavoprotein autofluorescence. By varying the ADP or ATP concentration in flow chamber, we determined that OxPhos has a low affinity in cardiomyocytes. The experiments were repeated in a fluorometer on cardiomyocyte suspensions leading to similar autofluorescence changes induced by ADP as recorded under the microscope. ATP stimulated OxPhos more in a fluorometer than under the microscope, which was attributed to accumulation of ADP in fluorometer chamber. By calculating the flow profile around the cell in the microscope chamber and comparing model solutions to measured data, we demonstrate that intracellular structures impose significant diffusion obstacles in rat cardiomyocytes.