Simson P, Jepihhina N, Laasmaa M, Peterson P, Birkedal R, Vendelin M
J. Mol. Cell. Cardiol. Volume 97, August 2016, Pages 197–203;
PMID: 27261153
Full text: http://doi.org/10.1016/j.yjmcc.2016.04.012
Abstract
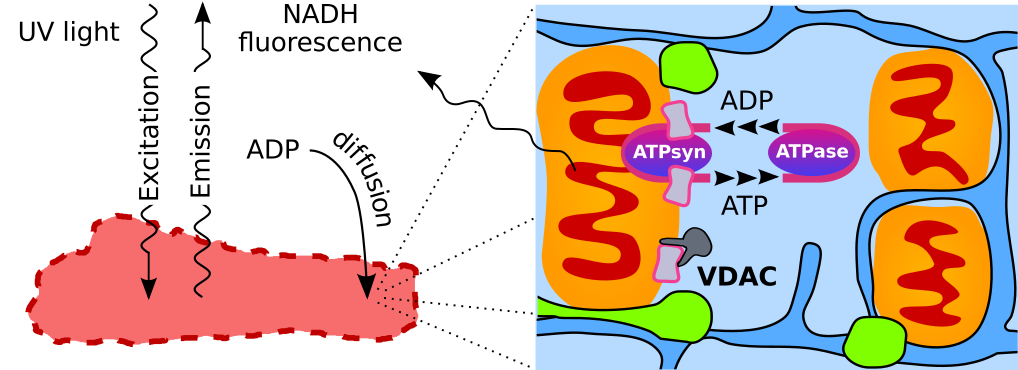
Adequate intracellular energy transfer is crucial for proper cardiac function. In energy starved failing hearts, partial restoration of energy transfer can rescue mechanical performance. There are two types of diffusion obstacles that interfere with energy transfer from mitochondria to ATPases: mitochondrial outer membrane (MOM) with voltage-dependent anion channel (VDAC) permeable to small hydrophilic molecules and cytoplasmatic diffusion barriers grouping ATP-producers and -consumers. So far, there is no method developed to clearly distinguish the contributions of cytoplasmatic barriers and MOM to the overall diffusion restriction. Furthermore, the number of open VDACs in vivo remains unknown. The aim of this work was to establish the partitioning of intracellular diffusion obstacles in cardiomyocytes. We studied the response of mitochondrial oxidative phosphorylation of permeabilized rat cardiomyocytes to changes in extracellular ADP by recording 3D image stacks of NADH autofluorescence. Using cell-specific mathematical models, we determined the permeability of MOM and cytoplasmatic barriers. We found that only ~2% of VDACs are accessible to cytosolic ADP and cytoplasmatic diffusion barriers reduce the apparent diffusion coefficient by 6-10×. In cardiomyocytes, diffusion barriers in the cytoplasm and by the MOM restrict ADP/ATP diffusion to similar extents suggesting a major role of both barriers in energy transfer and other intracellular processes.